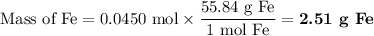Answer:
The amount of Iron that participated in the chemical reaction was 2,51g
Step-by-step explanation:
First: We look out for the atomic weight of Iron, as we can see in the image below, its atomic weight is 55.845 g/ mol
Second: Now, we multiply the amoung of moles with the atomic weight in order to cancel units of moles :