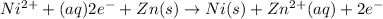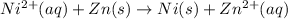Answer:
Overall balanced redox reaction :
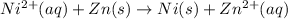
Step-by-step explanation:
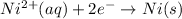 ..(1)
..(1)
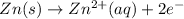 ..(2)
..(2)
Oxidation reaction is a chemical reaction in which an atom looses its electrons. Here, oxidation state of the atom increases.
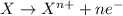
Reaction number 2 is an oxidation half reaction, in which zinc is loosing 2 electrons and forms zinc ion with +2 charge.
Reduction reaction is a chemical reaction in which an atom gains electrons. Here, the oxidation state of the atom decreases.
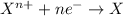
Reaction number 1 is a reduction half reaction, in which nickel ion with 2+ charge is gaining 2 electrons and forms nickel atoms.
Overall balanced redox reaction can be written adding (1) and (2):