Answer:
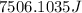
Step-by-step explanation:
We can use the relation
Q =mcΔθ
to find the energy required to heat the iron nail, where Q is the heat energy required, m is the mass of the nail and Δθ is change in temperature and c is heat capacity of the iron nail.
Also, from the question,
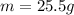
c = 0.4494J/g°C
Δθ=720 - 65 = 655°C
By substitution
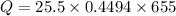
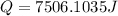
Hence the energy required to heat the iron nail is 7506.1035J