Answer: The molarity of the base solution is 0.22 M.
Step-by-step explanation:
According to the neutralization law,
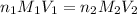
where,
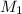 = molarity of
= molarity of
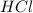 solution = 0.1994 M
solution = 0.1994 M
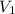 = volume of
= volume of
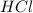 solution = 38.87 ml
solution = 38.87 ml
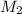 = molarity of
= molarity of
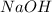 solution = ?
solution = ?
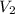 = volume of
= volume of
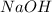 solution = Final reading - Initial reading = 36.18 - 1.16 = 35.02 ml
solution = Final reading - Initial reading = 36.18 - 1.16 = 35.02 ml
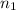 = valency of
= valency of
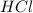 = 1
= 1
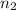 = valency of
= valency of
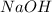 = 1
= 1
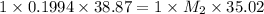
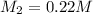
Therefore, the molarity of the base solution is 0.22 M.