Answer: The final rate will become 9 times of the initial rate.
Explanation: Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
![Rate=k[A]^x[B]^y](https://img.qammunity.org/2020/formulas/chemistry/middle-school/wq5ji9hbq4g6rx67qjbipwqn72erwdi258.png)
k= rate constant
x = order with respect to A
y = order with respect to B
n = x+y = Total order
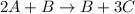
Rate law :
![Rate=k[A]^0[B]^2](https://img.qammunity.org/2020/formulas/chemistry/college/6nauxlnimupkzo3bro968tq56ynw7s1phw.png) , order with respect to A is 0, order with respect to B is 2 and total order is 2.
, order with respect to A is 0, order with respect to B is 2 and total order is 2.
Given: doubling the concentration of A and increasing the concentration of B by a factor of three.
![Rate'=[tex]k[2A]^0[3B]^2](https://img.qammunity.org/2020/formulas/chemistry/college/jgds8plnpe4tcpm8g60l0zer48opdao2kv.png)
![Rate'=k[2]^0[A]^0[3]^2[B]^2](https://img.qammunity.org/2020/formulas/chemistry/college/u310ffrsfl7peygn7i2qmup4gfvnsdy6de.png)
![Rate'=k* 1* [A]^0* 9* [B]^2](https://img.qammunity.org/2020/formulas/chemistry/college/mo4viscn3fuaqug4cg4spe5wd6nxk6gfgx.png)
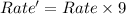
Thus the final rate will become 9 times of the initial rate.