Answer:
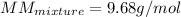
Step-by-step explanation:
Hello!
In this case, since the molar mass of gaseous mixtures can be determined via a weighted average containing the molar mass of each gas and the mole fraction (mole percent divided by 100) as shown below:
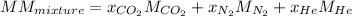
Now, we plug in to obtain:
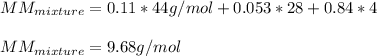
Best regards!