Step-by-step explanation:
Law of conservation of mass states that mass can neither be created nor it can be destroyed but it can be transformed into one form to another.
Similarly, law of conservation of energy states that energy can neither be created nor it can be destroyed as it can only be transformed from one form to another.
In modern view of matter and energy, is the law of mass conservation still relevant to chemical reactions as follows.
For example,
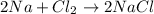
Atomic mass of Na = 23
Atomic mass of Cl = 35
Hence, mass of total number of reactants is calculated as follows.
[(2 \times 23) + (35 \times 2)] g/mol = 116 g/mol
Mass of total number of products is calculated as follows.
[2 \times (23 + 35)] = 116 g/mol
Thus, it is proved that in our modern view of matter and energy, is the law of mass conservation still relevant to chemical reactions.