Answer:
t = 1456.8 sec
Step-by-step explanation:
given data:
contant k = 2.60*10^{-6}
rate of crystallization is 0.0013 s-1
rate of transformation is given by
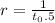
use specifies value to solve
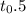
it is ime required for 50% tranformation
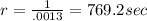
Avrami equation is given by
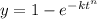

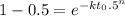
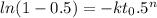
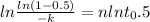
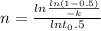
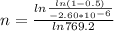
n = 1.88
second degree of recrystalization may be determine by rearranging original avrami equation
![t = [(-ln(1-y))/(k)]^(1/n)](https://img.qammunity.org/2020/formulas/engineering/college/ikkvwhz9mral33hkg6hq3m4qx0gqh2wl70.png)
for 90%completion
![t = [(-ln(1-0.9))/(2.60*10^(-6))]^(1/1.88)](https://img.qammunity.org/2020/formulas/engineering/college/oekmeux0r70r7fy205s0ctyec2a6vjs19s.png)
t = 1456.8 sec