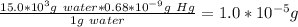Answer:
Quantity of Hg in 15.00 L water = 1.0*10^-5 g
Step-by-step explanation:
Given:
Concentration of Hg = 0.68 parts per billion (ppb)
Volume of water = 15.0 L
Density of water = 0.998 g/ml
To determine:
The amount of Hg in 15.0 L of water
Calculation:
1 ppb = 1 nanogram (ng) solute/1 g water
1 ng = 10⁻⁹ g
0.68 ppb = 0.68 ngHg/1 g water = 0.68*10⁻⁹g Hg/ 1g water
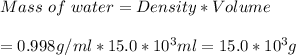
The amount of Hg in the above mass of water would be:
=