Answer:
(3) 345 nm
Step-by-step explanation:
Given:
Average C-C bond energy = 347 kJ/mol
To determine:
Wavelength of photon that can break a C-C bond
Calculation:
The energy (E) of a photon is related to its wavelength (λ) by the Planck's equation:
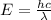
where h = Planck's constant = 6.626*10⁻³⁴ Js
c = speed of light = 3*10⁸ m/s
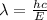
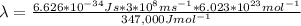
λ = 3.45*10⁻⁷ m
Since 1 nanometer (nm) = 10⁻⁹ m
The calculated wavelength corresponds to 345 nm