Answer:
Ethanoic or acetic acid.
Step-by-step explanation:
Hello!
In this case, since the chemical formula of the compound excludes the hydrogen from the CHO form, we can infer it is a leaving group, which is likely to be bonded with an oxygen due to the high polarity of the O-H bond. In such a way, the most feasible condensed structure is:
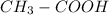
Whereas the COOH stands for the carboxyl group; therefore the compound is ethanoic acid or acetic acid.
Regards!