Answer:
Carbon dioxide have higher temperature at the final stage.
Step-by-step explanation:
Given that
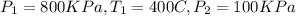
We know that
γ=1.28 for carbon dioxide
γ=1.4 for air
For isentropic expansion
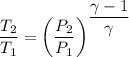
Now for carbon dioxide γ=1.28
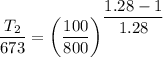
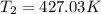
Now for air γ=1.4
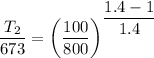
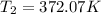
So carbon dioxide have higher temperature at the final stage.