Answer: The mass of tetraphosphorus decaoxide formed is 64.81g
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 ....(1)
....(1)
Given mass of potassium chlorate = 93.3 g
Molar mass of potassium chlorate = 122.55 g/mol
Putting values in above equation, we get:
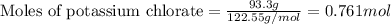
For the given chemical reaction:
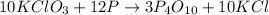
Red phosphorus is given in excess . So, it is considered as an excess reagent and potassium chlorate is considered as a limiting reagent.
By Stoichiometry of the reaction:
10 moles of potassium chlorate reacts with 3 moles of tetraphosphorus decaoxide
So, 0.761 moles of potassium chlorate will react with =
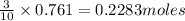 of tetraphosphorus decaoxide
of tetraphosphorus decaoxide
Calculating the mass of tetraphosphorus decaoxide by using equation 1, we get:
Molar mass of tetraphosphorus decaoxide = 283.886 g/mol
Moles of tetraphosphorus decaoxide = 0.2283 moles
Putting values in equation 1, we get:

Hence, the mass of tetraphosphorus decaoxide formed is 64.81g