Step-by-step explanation:
It is known that relation between equilibrium pressure and equilibrium partial pressure is as follows.
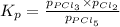
Initial pressures are given for
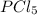 ,
,
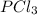 , and
, and
 at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
Substituting these values into the above formula as follows.
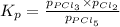
=
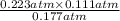
= 0.139 atm
or, = 0.140 (approx)
So,
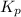 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of
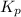 . Therefore, reaction will proceed in the forward reaction.
. Therefore, reaction will proceed in the forward reaction.
Therefore, partial pressure of
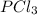 and
and
 needs to increase and
needs to increase and
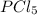 needs to decrease.
needs to decrease.