Step-by-step explanation:
The term
 means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.
means change in entropy. As entropy means the measure of randomness. This means that more randomly molecules of a substance are moving more will be its entropy.
If value of
 is negative then it means there is decease in entropy. When value of
is negative then it means there is decease in entropy. When value of
 is positive then it means there is increase in entropy.
is positive then it means there is increase in entropy.
In solids, molecules are closer to each other. So, entropy is minimum. Liquids has more entropy than solids and gases has maximum entropy.
- In the reaction,
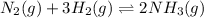 , total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of
, total 4 moles of gases form 2 mole of a gas. This means there is decrease in number of moles. As a result, there will be decrease in entropy. So, sign of
 is negative.
is negative.
- In the reaction,
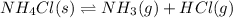 , total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of
, total 1 mole of solid substance is forming total 2 moles of gases. That is, there is increase in entropy. So, sign of
 is positive.
is positive.
- In the reaction,
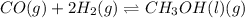 , total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of
, total 2 moles of gases are forming total 1 mole of liquid methanol. That is, there is decrease in entropy. So, sign of
 is negative.
is negative.
- In the reaction,
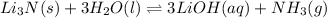 , total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of
, total 2 moles of substance is forming total 4 moles of substance. That is, there is increase in entropy so, sign of
 is positive.
is positive.