Given:
Pressure, P = 1.3 bar
Temperature, T = 500 K
velocity, v = 40 m/s
Pressure, P' = 0.85 bar
velocity, v' = 250 m/s
k = 1.4
Solution:
Now, we know that:
specific heat at constant pressure,
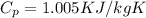
specific heat at constant volume,
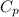 = 1.005 KJ/kgK
= 1.005 KJ/kgK
k =
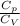
(a) To calculate temperature at exit, T'
Using steady flow Eqn:
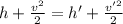 (1)
(1)
where
h = enthalpy =
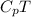
h'=
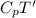
Now, from eqn (1)-
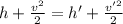
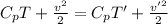
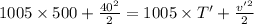
T' = 469.70 K
(b) To calculate % isentropic nozzle efficiency:
Using the relation:
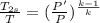
⇒
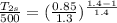
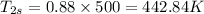
Now,
% isentropic nozzle efficiency,
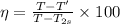
%
 %
%
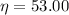 %
%