Answer: 2.17 g of bromide product would be formed
Step-by-step explanation:
The reaction of calcium bromide with lithium oxide will be:
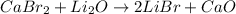
To calculate the moles :
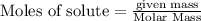
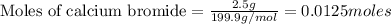
As lithium oxide is in excess, calcium bromide is the limiting reagent.
According to stoichiometry :
1 mole of
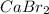 produce = 2 moles of
produce = 2 moles of
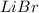
Thus 0.0125 moles of
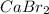 will require=
will require=
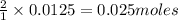 of
of
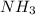
Mass of
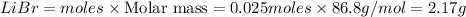
Thus 2.17 g of bromide product would be formed