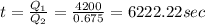Answer:
6222.22 sec
Step-by-step explanation:
Given data the power input to the refrigerator is 450 W
The COP of refrigerator is 1.5
Temperature
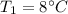
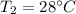
mass of watermelon =10 kg
specific heat =4.2 KJ/kg°C
The amount of heat removed from 5 watermelon
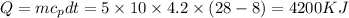
We know that
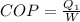
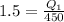
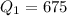 W=0.675 KW
W=0.675 KW
so time required to cool the watermelon is