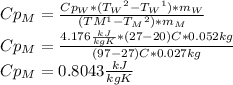Answer:
The heat capacity of the metal is:
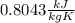
Step-by-step explanation:
Consider the energy balance; it says that the energy in the initial state is equal to the energy in the final state for the system, where the system is the water and the metal. M and W subscripts mean metal and water respectively, and 1 and 2 superscripts means the initial an the final state respectively.
The energy balance is:
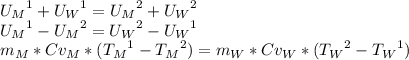
The difference between Cp and Cv is neglective for solids and liquids, so it is possible to change Cv by Cp:
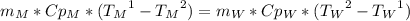
And the only unknown from the equation is
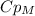 , so:
, so: