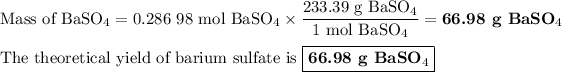Answer:
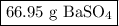
Step-by-step explanation:
We know we will need an equation with masses and molar masses, so let’s gather all the information in one place.
M_r: 261.34 233.39
Ba(NO₃)₂ + Na₂SO₄ ⟶ BaSO₄ + 2NaNO₃
m/g: 75.00
1. Moles of Ba(NO₃)₂
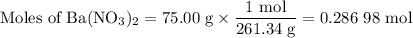
2. Moles of BaSO₄
The molar ratio is (1 mol BaSO₄/1 mol Ba(NO₃)₂
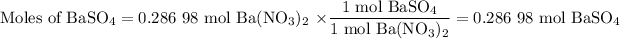
3. Mass of BaSO₄