Answer : The concentration of
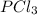 and
and
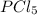 at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M
Explanation :
First we have to calculate the concentration of
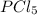 .
.
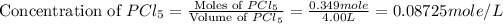
The given equilibrium reaction is,
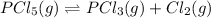
Initial conc. 0.08725 0 0
At equilibrium (0.08725-x) x x
The expression for equilibrium constant will be,
![K_c=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/gpkvotujabdxursdhkqb051q385dj25ify.png)
Now put all the given values in this expression, we get:
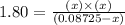
By solving the term 'x', we get:
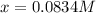
The concentration of
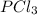 at equilibrium = x = 0.0834 M
at equilibrium = x = 0.0834 M
The concentration of
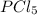 at equilibrium = (0.08725-x) = (0.08725-0.0834) = 0.00385 M
at equilibrium = (0.08725-x) = (0.08725-0.0834) = 0.00385 M
Therefore, the concentration of
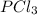 and
and
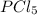 at equilibrium are, 0.0834 M and 0.00385 M
at equilibrium are, 0.0834 M and 0.00385 M