Answer:
Vapour pressure of solution is 78.151 torr
Step-by-step explanation:
Molar mass of biphenyl = 154.21 g
Molar mass of benzene = 78.11 g
19.2 g biphenyl = (19.2/154.21) moles of biphenyl = 0.125 moles of biphenyl
33.7 g of benzene = (33.7/78.11) moles of benzene = 0.431 moles of benzene
Total number of moles = (0.125+0.431) moles = 0.556 moles
Mole fraction of benzene in solution = (0.431/0.556) = 0.775
According to Roults law, vapour pressure of solution made from non-volatile solute =
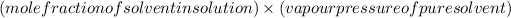
Here solute is biphenyl and solvent is benzene
So, vapour pressure of solution =
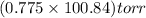 = 78.151 torr
= 78.151 torr