Answer: half life =
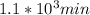 ,
,
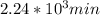 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and
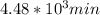 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% .
Explanation: The given information says, the reaction is first order with respect to
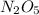 . For first order reaction, rate constant and half life are related to each other as:
. For first order reaction, rate constant and half life are related to each other as:
half life =
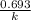
(where k stands for rate constant)
Value of k is given as
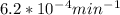
half life =
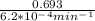
half life = 1117.74 min or
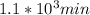
Let's say the initial concentration is 100. It asks to calculate the time taken to decrease the concentration to 25% which will be 25 as we have taken the initial concentration 100.
The equation that we use is:
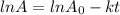
Where,
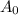 is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
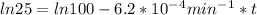
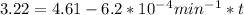
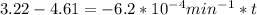
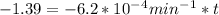
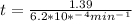
t = 2241.94 min or
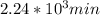
We can calculate the time when the concentration decreases to 6.25% of its initial value same as we did for the above.
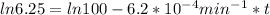
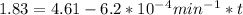
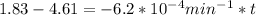
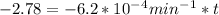
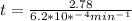
t = 4483.87 min or
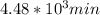
So, the answers for all the three parts are: half life =
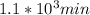 ,
,
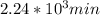 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and
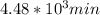 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% .