Answer: The pH of the cleaning compound is 10.44
Step-by-step explanation:
pH is defined as the negative logarithm of hydrogen or hydronium ion concentration that are present in a solution.
The equation representing pH of the solution follows:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
We are given:
![[H^+]=3.6* 10^(-11)](https://img.qammunity.org/2020/formulas/chemistry/high-school/r7putz1ztwtl32e4879jp5q82ac1l7woe7.png)
Putting values in above equation, we get:
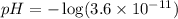
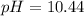
Hence, the pH of the cleaning compound is 10.44