Answer : The percent-by-mass concentration of acetic acid is, 5.15 %
Explanation : Given,
Mass of acetic acid = 51.80 g
Density of solution = 1.005 g/ml
Volume of solution = 1.000 L = 1000 ml (1 L = 1000 ml)
First we have to calculate the mass of solution.
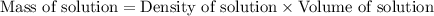
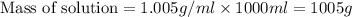
Now we have to calculate the percent-by-mass concentration of acetic acid.
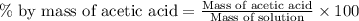
Now put all the given values in this formula, we get:
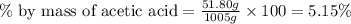
Therefore, the percent-by-mass concentration of acetic acid is, 5.15 %