Answer:
 = 42.9 atm,
= 42.9 atm,
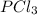 = 93.4 atm and
= 93.4 atm and
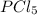 = 7.66 atm
= 7.66 atm
Explanation: The given balanced equation is:
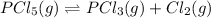
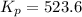
Initial pressure of
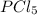 = 50.560 atm
= 50.560 atm
initial pressure of
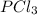 = 50.500 atm
= 50.500 atm
Let's say the change in pressure is p. Then:
equilibrium partial pressure of
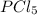 = (50.560 - p) atm
= (50.560 - p) atm
equilibrium partial pressure of
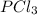 = (50.500 + p) atm
= (50.500 + p) atm
equilibrium partial pressure of
 = p atm
= p atm
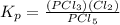
Let's plug in the values in it:
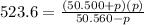
on cross multiply:
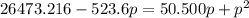
on rearranging the above equation:
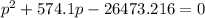
It's a quadratic equation. On solving this equation:
p = 42.9
So, the equalibrium partial pressure of
 = 42.9 atm
= 42.9 atm
equilibrium partial pressure of
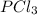 = 50.500 + 42.9 = 93.4 atm
= 50.500 + 42.9 = 93.4 atm
equilibrium partial pressure of
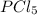 = 50.560 - 42.9 = 7.66 atm
= 50.560 - 42.9 = 7.66 atm