Answer:
Quality of vapor is equal to 17.3%.
Step-by-step explanation:
We know that if we know only one property in side the wet region then we will find the other property by using steam property table.
So pressure at saturation temperature 20°C
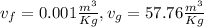
So specific volume v
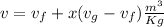
Where x is quality of mixture
Now putting the values ,given that
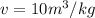
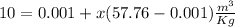
x=0.173
So quality of vapor is equal to 17.3%.