Answer:
(d) 2996 kJ
Step-by-step explanation:
We have given initial volume
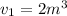
initial pressure
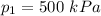
initial temperature
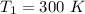
we know that during isothermal process the work done is given by
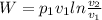
where
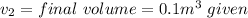
putting all these value in formula of work done

=-2995.8 kJ here negative sign indicates that work is dine on the gas
so wok done =2995.8 kJ