Answer:
0.425 moles of P₂O₅.
Step-by-step explanation:
As per given balanced equation, four moles of phosphorus reacts with five moles of oxygen to give two moles of P₂O₅.
As given that the initial moles of phosphorus taken = 0.97 moles
moles of phosphorus left after reaction = 0.12 moles
moles of phosphorus reacted = 0.97-0.12 = 0.85 moles
When four moles of P reacts they give two moles of P₂O₅.
when one mole of P will react to give =
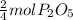
0.85 moles of P will react to give =
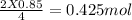 P₂O₅
P₂O₅