Answer:
Part a)
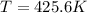
Part b)
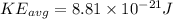
Part c)
in order to find the average speed we need to know about the the gas molar mass or we need to know which gas it is.
Step-by-step explanation:
Part a)
As per ideal gas equation we know that
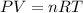
here we know that
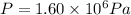
n = 3.80 moles
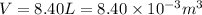
now from above equation we have
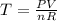
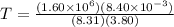
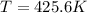
Part b)
Average kinetic energy of the gas is given as
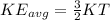
here we know that
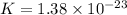
T = 425.6 K
now we have
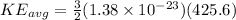
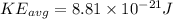
Part c)
in order to find the average speed we need to know about the the gas molar mass or we need to know which gas it is.