Answer:
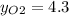 %
%
Step-by-step explanation:
The ethanol combustion reaction is:
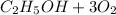 →
→
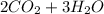
If we had the amount (x moles) of ethanol, we would calculate the oxygen moles required:
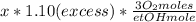
Dividing the previous equation by x:
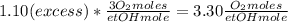
We would need 3.30 oxygen moles per ethanol mole.
Then we apply the composition relation between O2 and N2 in the feed air:
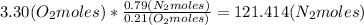
Then calculate the oxygen moles number leaving the reactor, considering that 0.85 ethanol moles react and the stoichiometry of the reaction:
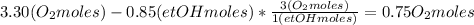
Calculate the number of moles of CO2 and water considering the same:
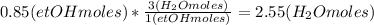
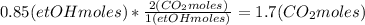
The total number of moles at the reactor output would be:
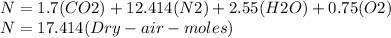
So, the oxygen mole fraction would be:
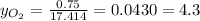 %
%