Answer:
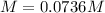
Step-by-step explanation:
Hello,
In this case, the molarity is defined as the ratio between the moles of the solute and the volume of the solution in liters:
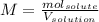
Thus, the moles of the solute which is magnesium chloride are:
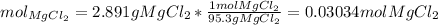
Nonetheless, the volume of the solution should be computed by adding the mass of both calcium chloride and water as we know the density of the solution as shown below:
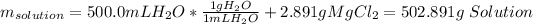
Hence, the volume is:
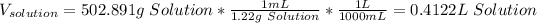
Finally, the molarity results:
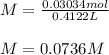
Best regards.