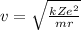Answer:
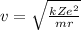
Step-by-step explanation:
The electrostatic attraction between the nucleus and the electron is given by:
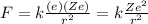 (1)
(1)
where
k is the Coulomb's constant
Ze is the charge of the nucleus
e is the charge of the electron
r is the distance between the electron and the nucleus
This electrostatic attraction provides the centripetal force that keeps the electron in circular motion, which is given by:
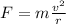 (2)
(2)
where
m is the mass of the electron
v is the speed of the electron
Combining the two equations (1) and (2), we find
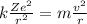
And solving for v, we find an expression for the speed of the electron: