Answer:
For methanol: Heat of combustion = -22.6 kJ / 0.0312 moles = -724.3590 kJ/mol (negative sign signifies release of heat)
For ethanol: Heat of combustion = -29.7 kJ / 0.0217 moles = -1368.6636 kJ/mol (negative sign signifies release of heat)
For propanol: Heat of combustion = -33.4 kJ / 0.0166 moles = -2012.0482 kJ/mol (negative sign signifies release of heat)
Step-by-step explanation:
Given:
Mass of Methanol = 1.0 g
Mass of ethanol = 1.00 g
Mass of n-propanol = 1.00 g
For methanol:
2 CH₃OH + 3 O₂ ----> 2 CO₂ + 4 H₂O, ∆H₀ = -22.6 kJ/g (negative sign signifies release of heat)
1 g of methanol on combustion gives 22.6 kJ of energy
Calculation of moles of methanol:
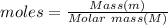
Molar mass of methanol = 32.04 g/mol
Thus moles of methanol = 1 g/ (32.04 g/mol) = 0.0312 moles
Hence energy in kJ/mol:
Heat of combustion = -22.6 kJ / 0.0312 moles = -724.3590 kJ/mol (negative sign signifies release of heat)
For ethanol:
C₂H₅OH + 3 O₂ ----> 2 CO₂ + 3 H₂O, ∆H₀ = -29.7 kJ/g (negative sign signifies release of heat)
1 g of ethanol on combustion gives 29.7 kJ of energy
Calculation of moles of ethanol:
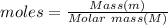
Molar mass of ethanol = 46.07 g/mol
Thus moles of ethanol = 1 g/ (46.07 g/mol) = 0.0217 moles
Hence energy in kJ/mol:
Heat of combustion = -29.7 kJ / 0.0217 moles = -1368.6636 kJ/mol (negative sign signifies release of heat)
For propanol:
2 C₃H₇OH + 9 O₂ ----> 6 CO₂ + 8 H₂O, ∆H₀ = -33.4 kJ/g , (negative sign signifies release of heat)
1 g of methanol on combustion gives 33.4 kJ of energy
Calculation of moles of methanol:
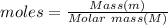
Molar mass of methanol = 60.09 g/mol
Thus moles of methanol = 1 g/ (60.09 g/mol) = 0.0166 moles
Hence energy in kJ/mol:
Heat of combustion = -33.4 kJ / 0.0166 moles = -2012.0482 kJ/mol (negative sign signifies release of heat)