Given:
Lattice parameter, a =
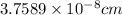
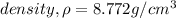
Solution:
We know that for FCC, total no. of atoms in a crystal lattice = 4
let the number of atoms in Tin for alloying be 'n'
⇒ Total no. of Copper atoms in the alloy, = 4 - n
Also mass of Copper, m = 63.54 g/mol
atomic mass of Tin = 118.69 g/mol
We Know density of the crystal lattice is given by the formula:
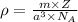 (1)
(1)
where,
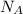 = Avagadro's number =
= Avagadro's number =
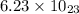
Putting all the values in eqn (1), we get
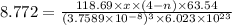
280.6 = 55.15n +254.16
n = 0.479 atoms/cell
Now to calculate the atomic percentage of Tin present in the alloy:
atomic percentage =
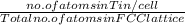
atomic % Tin present in alloy =
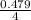 =
=
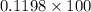
atomic % Tin present in alloy = 11.98%