Solution:
Given :
atomic radius, r = 0.1363nm = 0.1363×10⁻⁹m
atomic wieght, M = 95.96
Cell structure is BCC (Body Centred Cubic)
For BCC, we know that no. of atoms per unit cell, z = 2
and atomic radius, r =
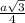
so, a =
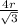
m = mass of each atom in a unit cell
mass of an atom =
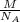 ,
,
where,
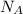 is Avagadro Number = 6.02×10^{23}
is Avagadro Number = 6.02×10^{23}
volume of unit cell = a^{3}
density, ρ =
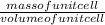
density, ρ =
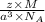
ρ =
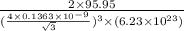
ρ = 10.215gm/
