Answer : The concentration of
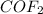 remains at equilibrium will be, 0.37 M
remains at equilibrium will be, 0.37 M
Explanation : Given,
Equilibrium constant = 4.90
Initial concentration of
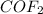 = 2.00 M
= 2.00 M
The balanced equilibrium reaction is,
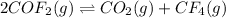
Initial conc. 2 M 0 0
At eqm. (2-2x) M x M x M
The expression of equilibrium constant for the reaction will be:
![K_c=([CO_2][CF_4])/([COF_2]^2)](https://img.qammunity.org/2020/formulas/chemistry/college/3x1u8agoi637qnmztu55adzmhx8wojsqqb.png)
Now put all the values in this expression, we get :
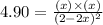
By solving the term 'x' by quadratic equation, we get two value of 'x'.
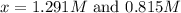
Now put the values of 'x' in concentration of
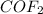 remains at equilibrium.
remains at equilibrium.
Concentration of
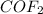 remains at equilibrium =
remains at equilibrium =
![(2-2x)M=[2-2(1.219)]M=-0.582M](https://img.qammunity.org/2020/formulas/chemistry/college/11ddc2kyy7tiidf3vowx578uf7wqitsy1t.png)
Concentration of
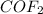 remains at equilibrium =
remains at equilibrium =
![(2-2x)M=[2-2(0.815)]M=0.37M](https://img.qammunity.org/2020/formulas/chemistry/college/hpmziywzv3ccrrjk3c2c12e2ecz33lmy6z.png)
From this we conclude that, the amount of substance can not be negative at equilibrium. So, the value of 'x' which is equal to 1.291 M is not considered.
Therefore, the concentration of
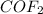 remains at equilibrium will be, 0.37 M
remains at equilibrium will be, 0.37 M