Step-by-step explanation:
(a)
The measure of material's ability to conduct thermal energy (heat) is known as thermal conductivity. For examples, metals have high thermal conductivity, it means that they are very efficient at conducting heat. The SI unit of heat capacity is W/m.K.
The expression for thermal conductivity is:
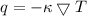
Where,
q is the heat flux
 is the thermal conductivity
is the thermal conductivity
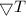 is the temperature gradient.
is the temperature gradient.
(b)
Heat capacity for a substance is defined as the ratio of the amount of energy required to change the temperature of the substance and the magnitude of temperature change. The SI unit of heat capacity is J/K.
The expression for Heat capacity is:
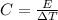
Where,
C is the Heat capacity
E is the energy absorbed/released
 is the change in temperature
is the change in temperature
(c)
Thermal diffusivity is defined as the thermal conductivity divided by specific heat capacity at constant pressure and its density. The Si unit of thermal diffusivity is m²/s.
The expression for thermal diffusivity is:
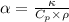
Where,
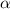 is thermal diffusivity
is thermal diffusivity
 is the thermal conductivity
is the thermal conductivity
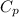 is specific heat capacity at constant pressure
is specific heat capacity at constant pressure
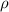 is density
is density