Solution:
Given:
pressure ratio,
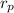 = 6.75
= 6.75
Formula used:
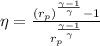
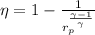 (1)
(1)
where,
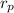 = pressure ratio
= pressure ratio
γ = specific heat ratio of a gas( here, helium gas it is 1.667)
Now,
Eqn (1 ) is for thermal efficiency of an ideal gas, using eqn (1), we get
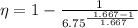
\eta = 1- \frac{1}{2.1469} = 0.5342
percentage thermal efficiency, \eta =53.42%