Answer:
Process 1:W=0
Process 2:W= -386.13 KJ
Process 3:W= -468 KJ
Step-by-step explanation:
Process 1:
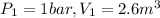
Process 2:
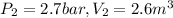
Process 3:
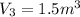
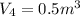
Process 1:
Work (W)=0 ,because it is constant volume process.
Process 2:
It is constant temperature process so PV=C
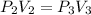
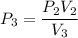
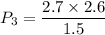
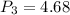 bar
bar
So work in constant temperature process
W=
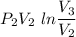
W=
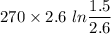 (1 bar=100KPa)
(1 bar=100KPa)
W= -386.13 KJ
Negative sign means it is compression process.
Process 3:
It is a constant pressure.
So work W=
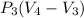
W=468(0.5-1.5) KJ
W= -468 KJ
Negative sign means it is compression process.