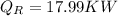Answer:
a)COP=5.01
b)
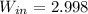 KW
KW
c)COP=6.01
d)
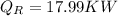
Step-by-step explanation:
Given
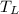 = -12°C,
= -12°C,
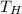 =40°C
=40°C
For refrigeration
We know that Carnot cycle is an ideal cycle that have all reversible process.
So COP of refrigeration is given as follows
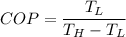 ,T in Kelvin.
,T in Kelvin.
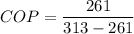
a)COP=5.01
Given that refrigeration effect= 15 KW
We know that
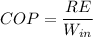
RE is the refrigeration effect
So
5.01=
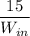
b)
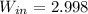 KW
KW
For heat pump
So COP of heat pump is given as follows
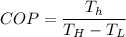 ,T in Kelvin.
,T in Kelvin.
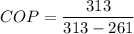
c)COP=6.01
In heat pump
Heat rejection at high temperature=heat absorb at low temperature+work in put
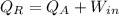
Given that
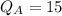 KW
KW
We know that
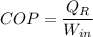
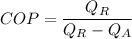
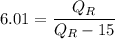
d)