Answer:
a) Control Volume
b) 0.44 kJ/kg/K
c) 4.18 kJ/kg/K
d) 32°C
Step-by-step explanation:
a. The system is a control volume because mass can be added and removed from the system.
b) Nickel has a heat capacity of 0.44 kJ/kgK
c) The heat capacity of water at standard conditions is 4.18 kJ/kgK
d)
The thermal equilibrium means that the system heat transfer of the nickel and the water are the same:
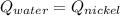
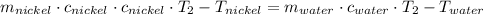
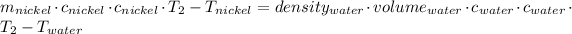
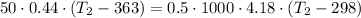
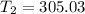
The temperature will be 305.03 K which is 32°C