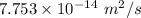Answer:
The value of D is
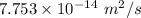
Step-by-step explanation:
Given that,
Temperature = 705°C
Maximum diffusion
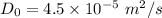
Activation energy
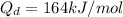
We need to calculate the value of D
Using formula of diffusion coefficient
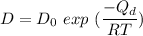
Where, D = diffusion coefficient
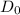 = Maximum diffusion coefficient
= Maximum diffusion coefficient
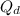 = Activation energy
= Activation energy
T = temperature
R = Gas constant
Put the value into the formula
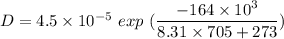
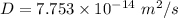
Hence, The value of D is