Answer:
a) Sp = 19.82 MeV
b) Sp = 15.96 MeV
c) Sp = 26.21240 MeV
Step-by-step explanation:
He atomic number = 2
He mass number = 4
He molar mass = 4.002602 u
Element with 2-1 atomic number = 1 and Element with mass number = 4-1 = 3 is Tritium
Tritium molar mass = 3.0160492 u
Hydrogen molar mass = 1.00784 u
separation energy
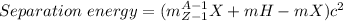
Sp=(3.0160492+1.00784-4.002602)(931.50 MeV/u) (1 u = 931.5 MeV)
a) Sp = 19.82 MeV
Carbon atomic number 6
Mass number = 12
Element with 6-1 atomic number = 5 and Element with mass number = 12-1 = 11 is Boron 11
Sp=(mass of boron 11+mass of hydrogen+mass of carbon)×931.5
Sp=(11.009305+1.00784-12)×931.5
b) Sp = 15.96 MeV
Calcium atomic number = 20
Calcium mass number = 40
Sp=(mass of potassium+mass of hydrogen+mass of calcium)×931.5
Sp=(39.0983+1.00784-40.078)×931.5
c) Sp = 26.21240 MeV