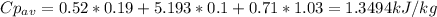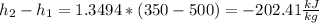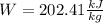Answer:
The work developed is 202.4
Step-by-step explanation:
First, write the energy balance equation:
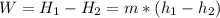
The enthalpy change for an ideal gas is just function of its temperature, so the pressure changes are not relevant; the enthalpy change of an ideal gas may be calculated as:
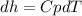
Assuming Cp constant,
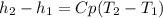
The average Cp is then calculated with data from van Wylen table A.6 with a simple mix rule based on molar fractions: