Answer:
Step-by-step explanation:
Given parameters:
Number of moles = 12moles
Temperature = 273K
Pressure = 75kPa, convert to atm:
1kPa = 0.00986923atm
75kPa = 75 x 0.00986923 = 0.74atm
Unknown:
Volume of the oxygen gas
Solution:
We simply apply the idea gas law which is :
PV = nRT
Since the unknown is volume, we make it the subject of the formula:
V =
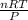
R is the gas constant and its value is given as 0.082
V =
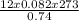 = 363.01L
= 363.01L