Answer:
The element produced is gallium, Ga
Step-by-step explanation:
Given:
Moles of the product = 3 moles
Weight of the product = 209.1 g
To determine:
The identity of the product formed i.e. the unknown element
Calculation:
The identity of element can be deduce from its atomic weight and comparing the calculated weight to that of the elements in the periodic table.
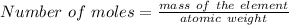
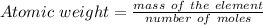
In this case:
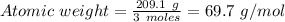
From the periodic table, the element with an atomic mass = 69.7 g/mol is gallium, Ga