Answer:500,551.02
Step-by-step explanation:
Given
Initial enthaly of pump \left ( h_1\right )=500KJ/kg
Final enthaly of pump \left ( h_2\right )=550KJ/kg
Final enthaly of pump when efficiency is 100%=
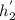
Now pump efficiency is 98%
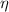 =
=
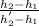
0.98=
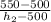
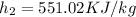
therefore initial and final enthalpy of pump for 100 % efficiency
initial=500KJ/kg
Final=551.02KJ/kg