Answer: The given statement is false.
Step-by-step explanation:
Precipitation reaction is defined as the chemical reaction in which two aqueous solution upon mixing together results in the formation of an insoluble solid.
For example,
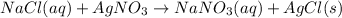
Here AgCl is present in solid state so, it is the precipitate.
But it is not necessarily true that two aqueous solutions will always result in the formation of a precipitate.
For example,
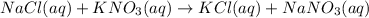
Hence, we can conclude that the statement precipitation reactions always occur when two aqueous solutions are mixed, is false.