Answer: 61 grams
Step-by-step explanation:
To calculate the number of moles, we use the equation:
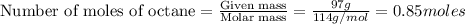
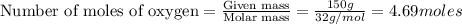
The chemical equation for the combustion of octane in oxygen follows the equation:
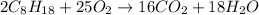
By stoichiometry of the reaction;
25 moles of oxygen react with 2 moles of octane
4.69 moles of oxygen react with=
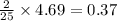 moles of octane
moles of octane
Thus, oxygen is the limiting reagent as it limits the formation of product and octane is the excess reagent.
25 moles of oxygen produce 18 moles of water
4.69 moles of oxygen produce=
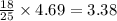 moles of water.
moles of water.
Mass of water produced=
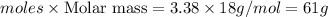
The maximum mass of water that could be produced by the chemical reaction is 61 grams.